When it comes to discussing the future of energy, there is the usual renewable vs fossil fuel debate. I already discussed that extensively in this article, as well as in the reports about CNOOC and RIG.
Another option that is regularly discussed is hydrogen. Hydrogen is often presented as the soon-to-be efficient technology that might even replace battery-powered electric vehicles (BEV).
In this article, I will try to explain quickly why using hydrogen for cars or for energy storage on the grid is just a terrible idea. And that no amount of extra R7D spending will solve it, as the problem is rooted in fundamental physics, chemistry, and thermodynamics.
How would a hydrogen economy work?
The first step to understand the topic is to realize that hydrogen is not a "primary" energy source, like coal, oil, solar, or uranium. There is no deposit of hydrogen ready to be tapped and exploited.
Hydrogen, a very energetic compound, has to be produced out of something else.
One option is to produce it out of hydrocarbon (generally methane), as the source material is energetic enough to provide the energy to produce hydrogen. Problem is that the process emits carbon, making such "blue" hydrogen far from useful from a global warming perspective. Better burn the gas directly.
Another option is to break apart water molecules. Two times H20 into 2x H2 (hydrogen gas) and 1x o2 (breathable oxygen). The preferred method for this is electrolysis.
Once the hydrogen is produced, it needs to be delivered to its point of consumption. Like a fuel station for example.
And then it needs to be used to perform the wanted task, generally generate electricity for the grid or for an electric vehicle.
Each of these steps requires massive capital investment to build the required infrastructure. This is the first obstacle, but one that could be overcome if the hydrogen economy was profitable.
So the idea is to use "green" power to produce hydrogen and used that hydrogen
Why hydrogen?
Before I go deeper and explain why it will not work, I will give a quick review of the good part of hydrogen:
It is not polluting. At least, its emission when used is only water, so this is
It is mostly carbon neutral if produced with renewable or nuclear energy,
It is much more energy-dense than batteries, so it has a better range and less dead weight.
It can be refueling a car in seconds, where batteries take 10-20 min.
It can be stored for a long period of time, in large quantities.
Sadly, it is so inefficient that all those advantages are nullified by energy losses (see below)
The chemistry problem
There is actually 2 set of problems with hydrogen. One chemical and one about thermodynamics.
The first problem is that hydrogen is a very small molecule. Hydrogen atoms are the smallest atoms, and that makes hydrogen an extremely light gas. It also means that as the atoms are so small, they are likely to leak through materials that would retain larger molecules like methane (a carbon atom is 12x heavier than a hydrogen atom).
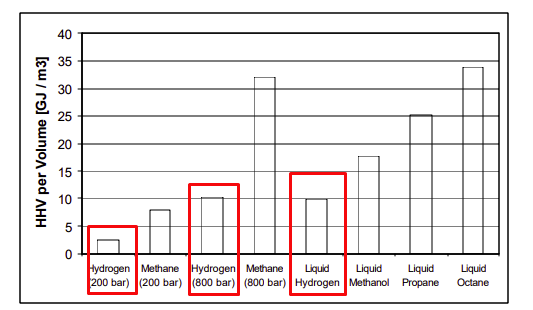
In gas form, it is simply too low-density stuff to be carried in that form. The gas tank would just be enormous. Or at enormous pressure, from 200-800 times the atmospheric pressure, which is a huge hazard and technical issue.
In liquid form, hydrogen is slightly more dense than pressured methane. This is not terrible, but a LOT less dense than oil or other liquid fuels.
https://afdc.energy.gov/files/pdfs/hyd_economy_bossel_eliasson.pdf
So you need to liquefy it. This means cooling it down at -252 Celsius / -423 F. This is low even by the liquefied gas standard. Again, hydrogen's very nature is to stay as a gas. Not an easy, cheap, or energy-free task.
You then need to keep this liquid hydrogen at that ultra-low temperature in a special cryogenic container.
It consumes a lot of the energy stockpiled in the hydrogen. And it requires yet more capital expenditure.
Another issue with hydrogen is that is a very energetic and very reactive chemical. In another word, it can go BOOM very easily. Remember the Hindenburg zeppelin? That is why we stopped using hydrogen to inflate balloons and zeppelins. There are ways to secure a hydrogen tank, but it adds weight. And again, cost.
Overall, hydrogen is hard to handle because it is a very small molecule. Nothing can help solve these issues that are rooted in fundamental quantic and chemical laws.
Hydrogen competitor
So far, I explain how hard it is to handle hydrogen. The important part now is to understand it is a process with a lot of steps:
produce green electricity
electrolyze water into hydrogen
liquefy the hydrogen
transport the liquid hydrogen
convert the hydrogen back into electricity to be used in the grid or on an engine
As you see, we start and end with electricity. Hydrogen is just a way to transport and stock electricity.
So the competitor of hydrogen is not gas or oil. It is power lines and batteries.
Lithium batteries have a 90-95% efficiency, so the competition is tough. And hydrogen is a terrible battery that leaks away most of the energy you put in it, as you will see below
Thermodynamic and losses
For the non-scientist, it is easy to imagine that any problem can be solved with enough R&D spending. But in fact, every process has a maximum efficiency threshold. This means that no matter how good a machine is, you always have something like 5-20% losses.
So usually, the best solution is the most straightforward. The more steps and hurdle, the more efficiency accumulate and compound on each other.
Green hydrogen implies the following steps and decreases in the initial energy (if you follow the link, you will see I tried to pick the most positive numbers):
"green electricity" = 100% (ignoring small losses here like Dc to AC and grid losses)
Electrolysis = 75% of the initial energy left
Liquefaction = 75% * 80% = 60% left
Storage = 60% * 87% = 52% left
Transportation = 52% * 96% = 50%
Combustion / energy production
(if burned in a modified combustion engine) 50 * 60% = 30%
(if used by a fuel cell) 50% * 50% = 25%
Please take note that most of the steps are at 80%+ efficiency. There is no one big major failure, except when it comes to the motor itself. So from electrolysis to transportation, we might see marginal improvement, but before it reaches a car or a power plant, the hydrogen will have already wasted half of the initial energy.
Combustion engines and fuel cells are also unlikely to go much above current yield. So 2/3 of the initial energy is wasted. In ideal conditions. 3/4 if not everything is optimal, like often in the real world
Imagine batteries that burn away 2/3 of the power you put in it. And cost billions of dollars in infrastructure and capex to do so. Do that sound like a good system?
Said in another way, if solar and wind are only too intermittent and viable only with a massive hydrogen economy tied to it, their "real" efficiency/cost are 3x worse. And that is before taking into account capital and labor costs for the whole hydrogen infrastructure. This fits the notion I had written in a previous article that storage and grid costs will represent 80% of the green transition costs. A sum entirely ignored by policymakers.
So whenever we hear in the press that solar/wind cost X/kWh, we actually should up this number x3-5 to take into account storage costs created by intermittency.
What hydrogen can be good for?
I made a very strong case for hydrogen never being an efficient transportation fuel or energy storage method. Nevertheless, is there absolutely no possible green hydrogen use case? Actually, I think there are 3 niches uses cases that make sense. So to be fair, I wanted to mention them to make clear that some green hydrogen might make economic sense.
Long-haul transportation
A big problem with batteries is that they weigh A LOT. While somewhat manageable for cars, more problematic for trucks, it is a death sentence for aeronautic applications. In addition, jet engines burn fuel and electricity just cannot do that.
Burning hydrogen is maybe the only path, with biofuels, to turn air transport carbon neutral. I am skeptical about its competitivity with biofuel, as it needs to redesign all the planes on Earth. But maybe it can work and at least not compete with food supply like "bio" ethanol does.
Potential shipping could be hydrogen-powered too I guess, even if for now, LNG ships seem a better bet.
Steelmaking
An important step in steelmaking is reducing the iron ore, using coking coal. I long believed it to be a step that made coal irreplaceable. Apparently, it can be done with hydrogen instead. So I can imagine a strong use case for green hydrogen here, in order for steelmakers to avoid carbon taxes and such. Fortescue Metals, a low-cost giant iron miner, selling lately at a discount and 17% dividends yield, is focused on making it a reality.
Energy export
The last case is for hydrogen to be used as the carrier from an energy-rich area to an energy-poor area.
"But wait, didn't you say the losses make it a terrible energy carrier?"
Yes, I did. But in some exceptional cases, an area might be ultra-rich in cheap and green energy, with a very little population around needing it. This is especially true for geothermal energy that can be abundant, cheap, and renewable all at once.
I am thinking for example of Iceland and the Kamchatka peninsula (I explain the energy potential of Kamchatka in this article). These regions have such an abundance of ultra-cheap energy that even when losing 2/3 of it in transport to the final market, it could still be profitable. An no one around to use it.
I could also easily imagine hydrogen-powered ships and planes refueling there on the cheap, before resuming their trip on the trans-Atlantic road or the Arctic.



They also mentioned the plan to use salt cavities to stock the excess hydrogen
One use case of hydrogen would be fuel-switching for companies that want to decarbonise
INEOS illustrated the case, they are establishing steam reformation units in their Grangemouth complex to produce blue hydrogen
Which would then be used as fuel and piped to their various units using their current pipeline system
Still less efficient than natural gas but carbon is persona non grata now…